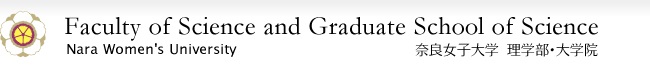Molecular Design for Fluorescent Metal Sensors
 [Release: December, 2015]
[Release: December, 2015]
Professor Yuji Mikata
Chemistry Course & Environmental Sciences Course
Department of Chemistry, Biology, and Environmental Science
Faculty of Science
Nara Women's University
The existence of enzymes is indispensable for supporting biological activities. Enzymes possess "substrate specificity", in which each enzyme selectively reacts with only a specific substance and makes specific biological reactions proceed efficiently. However, not just enzymes, but various other molecules inside our bodies rigorously distinguish among other molecules and metal ions within our bodies. By reproducing this "molecular recognition" event in a test tube, we can approach an "understanding of biological functions" that still holds many puzzles. With this aim, in my laboratory, we are performing research on the design and synthesis of molecules that can clearly distinguish various metal ions. Here, I introduce our latest research results in the development of fluorescent sensor molecules that can specifically identify zinc ion, which is involved with many biological activities and is an extremely important metal for our bodies, as well as cadmium and mercury ions, which are toxic heavy metals that causes severe pollution in the environment.
First, I will introduce a sensor that specifically identifies zinc ion. In my laboratory, we have focused on the compound named quinoline to synthesize various derivatives. We first synthesized TQEN in which four quinolines are bound to ethylenediamine (Fig. 1, Left). We found that TQEN emits fluorescence when it binds to a zinc ion to form a complex. However, in the case that TQEN binds to a cadmium ion, which is in the same group with zinc, it also exhibits a fluorescence with an intensity about 60% of that of zinc complex. Therefore, it was necessary to increase the specificity for zinc by modification of the molecular structure. When we examined the structure of the zinc complex of TQEN by X-ray crystallographic analysis, we learned that there was a large steric hindrance among the quinoline rings in the complex. Therefore, we concluded that the structure of the TQEN was not a suitable for the zinc ion binding; therefore, the specificity of fluorescent enhancement for the zinc ion was low. Then we synthesized 1-isoTQEN, in which the four "quinolines" of TQEN are replaced with "isoquinolines", and succeeded in eliminating the steric hindrance when the zinc complex was formed, so that it fit the zinc ion very well. Hence, we succeeded in developing a fluorescent sensor molecule that emits fluorescence specific to zinc ions (Fig. 1, Middle).
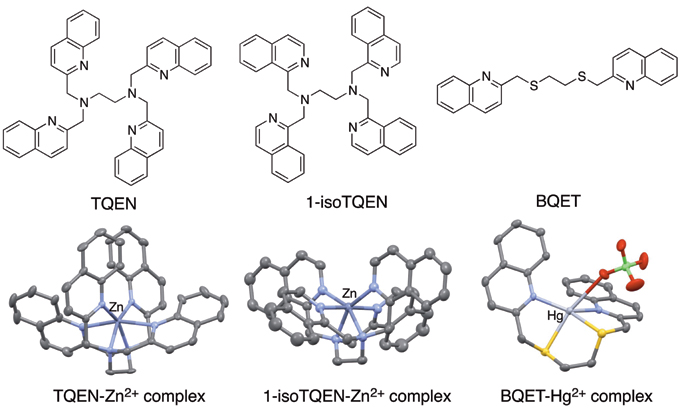
Figure 1. TQEN (Left), Zinc Ion Sensor 1-isoTQEN (Middle), Mercury Ion Sensor BQET (Right)
We also found that TQDACH, a compound replacing the ethylene portion of TQEN into cyclohexane moiety, is a zinc ion-specific fluorescent sensor (Fig. 2, Left). We then found that the compound TQPHEN, in which the cyclohexane of TQDACH is replaced by benzene, shows the completely opposite behavior, and is a cadmium ion-specific fluorescent sensor (Fig. 2, Right). That is, we found that a small change in the molecular structure makes a completely different metal selectivity in fluorescent response.
Thus, we succeeded in creating sensors to specifically recognize zinc and cadmium ions. Now we come to sensors for mercury ions. Based on the principle of chemistry that "like attracts like", we designed a mercury ion sensor. Specifically, to recognize mercury, which is a "soft atom" located at the bottom of the periodic table, we designed and synthesized BQET, which includes sulfur (S), as a "soft atom" instead of nitrogen (N) (Fig. 1, Right). Generally, heavy metals like mercury have a property called "quenching" (shut down the fluorescence of fluorophore), so developing a sensor that emits fluorescence upon binding with mercury is very difficult. However, our strategy in this research worked well, and we were successful in developing BQET, a sensor that emits fluorescence when it binds mercury.
As described above, based on the structure of TQEN, we developed sensor compounds for zinc, cadmium, and mercury ions. Notably, we found that just by making a small change to the structure of a molecule, its functions can dramatically change. We have been captured by the charm of chemistry, the idea that "you donít know until you try." We intend to discover molecules that exhibit new functions, and the students in my laboratory are engaged in synthesizing new compounds every day. Wonít you join us in creating new molecules for the world, to unlock the secrets of nature?
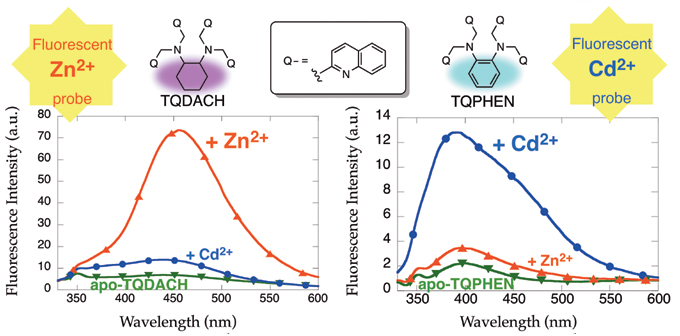
Figure 2. Zinc Ion Sensor TQDACH (Left) and Cadmium Ion Sensor TQPHEN (Right)
Recent Publications Related to this Research
(1) Y. Mikata, R. Ohnishi, A. Ugai, H. Konno, Y. Nakata, I. Hamagami, and S. Sato
OFF-ON-OFF fluorescent response of N,N,N',N'-tetrakis(1-isoquinolylmethyl)-2-hydroxy-1,3-propanediamine (1-isoHTQHPN) toward Zn2+
Dalton Trans., 45(17), 7250-7257 (2016).
http://pubs.rsc.org/en/content/articlelanding/2016/dt/c6dt00506c
(2) Y. Mikata, A. Kizu and H. Konno
TQPHEN (N,N,N',N'-tetrakis(2-quinolylmethyl)-1,2-phenylenediamine) derivatives as highly selective fluorescent probes for Cd2+
Dalton Trans., 44(1), 104-109 (2015).
http://pubs.rsc.org/en/content/articlelanding/2015/dt/c4dt02177k