Discovery of a "Switch" That Converts a Reaction Path
 [Release: February, 2016]
[Release: February, 2016]
Associate Professor Mikito Toda
Physics Course
Department of Mathematical and Physical Sciences
Faculty of Science
Nara Women's University
"Reaction paths follow the road over a saddle." Although this is a widely accepted idea, it needs to change because of a new phenomenon, whose existence we have recently predicted. The following is an outline of our research leading to this result and the future outlook for our research.
Studying Reaction Paths
Reaction processes are abundant in natural phenomena such as chemical and nuclear reactions. Mixing oxygen and hydrogen and igniting the mixture to produce water is an example of a chemical reaction. What path does such a reaction process follow? If the course of the reaction process is known, then its progress can be controlled, enabling us to accelerate or decelerate the process. Based on such motivations, we have been studying reaction paths.
Our initial guiding principle is the idea that "reaction paths follow the road over a saddle". First, let me explain what a "saddle" is. Today's younger people may not understand the importance of a saddle. A saddle is the lowest point in mountains, through which one can reach the other side of the mountain range that lies ahead. In today's world, where transportation by cars and trains is standard, a tunnel would be dug through a mountain if there iss a mountain ahead of your path. However, in the past, when people crossed a mountain on foot, the approach to reaching the other side with minimal effort (potential energy) was to follow the road through a saddle. For example, several saddles are available, such as the Dark Pass, along the roads leading from Nara to Osaka. Based on the same concept, the idea that "reaction paths follow the road over a saddle" is dominant today. This concept also supports the idea that a reaction path can be understood by analyzing the topography (potential surface) in which the reaction occurs. This is the theory known as the "Intrinsic Reaction Coordinate", proposed by Kenichi Fukui (who received a Nobel Prize in Chemistry for the frontier molecular orbital theory) and others.
However, this theory ignores the effect of the speed of the reaction. Analysis of the topography alone may be sufficient in the cases of a man walking across a mountain or a mountaineer considering the route for mountain climbing. On the other hand, in bobsled, a winter Olympics sport, the speed of the sled must be considered alongside the topography of the path to develop the sled's route. Similarly, there are cases in chemical reactions in which the impact of the speed cannot be ignored. Among such phenomena is the "switching of reaction paths", which we have predicted theoretically.
Switching the Reaction Path
When predicting a new phenomenon, it is preferable to work on as simple a system as possible. Thus, the system can be analyzed theoretically without uncertainties and future empirical validations will likely involve fewer difficulties. From this perspective, we have focused on the ionization of hydrogen atoms. We have analyzed the directional distribution at the point of ionization by first treating hydrogen atoms with an electrostatic field and a perpendicular static magnetic field, followed by an excitation to a high-excitation state by a laser. Because an energy wall is created during the ionization of electrons with the application of the electrostatic field, the hydrogen atoms are expected to follow the path of a saddle during the ionization process, according to the conventional theory. On the other hand, the static magnetic field exerted an additional force, proportional and vertical to the speed, to the electrons, changing the direction of their motions. This effect becomes particularly significant when the strength of the static magnetic field and the speed of electrons increase, finally causing the reaction path to switch to a direction perpendicular to that of the mountain pass.
Figure 1 shows a schematic of the concept. In the figure, a hydrogen atom (a proton and electron pair) is positioned at the bottom left. According to the conventional theory, the ionization of electrons follows the path of a saddle. This path is represented by a track that extends from the bottom left of the figure, crosses the pass, and descends to the other side of the mountain. The path perpendicular to the mountain pass is the reaction path after the switch. The reaction path switch is drawn as a turntable that changes between different tracks. However, figure 1 may cause misunderstandings. The new phenomenon that we have predicted does not occur in a two-dimensional topography, but only in a more than two-dimensional topography. To create a figure that includes the height of the mountain to be crossed, it must have at least four dimensions. Since such a figure cannot be created, our figure compromises accuracy to express the concept. This raises the question of why at least a three-dimensional topography is required for the predicted phenomenon. The key to answering this question is the concept of chaos.
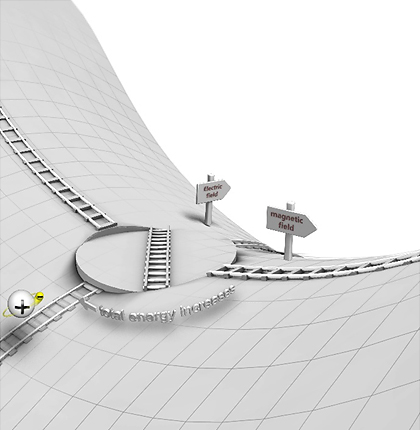
Fig. 1: Switch for a reaction path
What is Chaos?
Chaos is crucial to the research on reaction processes. This indicates the fact that our research is distinctly interdisciplinary. Chaos is a phenomenon first observed at the end of the 19th century by the French mathematician PoincarEduring his theoretical research on the solar system, which shows that a very complex motion that appears random can be actually described by a relatively simple equation. Chaos was later discovered in many other areas, including weather, biology, and economy, and is now accepted as a universal phenomenon observed across nature and society. It is essential that we also consider chaos when studying reaction paths. In figure 1, where the movement occurs in a two-dimensional topography, the direction perpendicular to the mountain pass is one-dimensional. On the other hand, in the case in which movement occurs in a three-dimensional topography, the direction perpendicular to the mountain pass is two-dimensional, and this is where chaos may occur. The presence of this chaos triggers the "switch in the reaction path".
Future Outlook
The new phenomenon that we have predicted may become important in non-equilibrium reaction processes induced by lasers and in chemical reactions in outer space. In recent years, it has been suggested that amino acids, which could be the source of life, may exist in space, and the study of chemical reactions in space (or space chemistry) is attracting interest. A challenge in applying our research in this context is the switch of reaction paths in quantum dynamics. Although our research is currently based on classical chaos, research on quantum chaos has progressed in recent years, leading to possibilities of new quantum phenomena, including the tunnel effect. Additionally, new quantum phenomena in the switching of reaction paths are possible and further developments can be expected in our future studies.
Acknowledgments
This research was performed jointly with assistant professor Hiroshi Teramoto and professor Tamiki Komatsuzaki of the Research Institute for Electronic Science at Hokkaido University, professor Masahiko Takahashi of the Institute of Multidisciplinary Research for Advanced Materials at Tohoku University, and professor Hirohiko Kono of the Tohoku University Graduate School of Science. The research was supported by the MEXT "Network Joint Research Center for Materials and Devices", which consists of Hokkaido University, Tohoku University, Tokyo Institute of Technology, Osaka University, and Kyushu University, and by the Kakenhi Scientific Research (C) & Challenging Research (Exploratory) grant from the Japan Society for the Promotion of Science. Findings from the research have been published at Physical Review Letters, a letters journal of the highest prestige in the field of physics, and its Editor encouraged us to write a press release. Given the encouragement, we held a press conference at Hokkaido University and Tohoku University, which was published by the press, such as the Nikkei Sangyo Shimbun. Figure 1 was used for the press release by the Hokkaido University and Tohoku University.
Papers related to this research follow.
(1)Mechanism and Experimental Observability of Global Switching Between Reactive and Nonreactive Coordinates at High Total Energies, Hiroshi Teramoto, Mikito Toda, Masahiko Takahashi, Hirohiko Kono, and Tamiki Komatsuzaki, Phys. Rev. Lett. 115, 093003, 2015
http://journals.aps.org/prl/abstract/10.1103/PhysRevLett.115.093003
(2)Breakdown mechanisms of normally hyperbolic invariant manifolds in terms of unstable periodic orbits and homoclinic/heteroclinic orbits in Hamiltonian systems,
Hiroshi Teramoto, Mikito Toda and Tamiki Komatsuzaki1,
Nonlinearity, Volume 28, Number 8 pp.2677-2698, 2015
http://iopscience.iop.org/article/10.1088/0951-7715/28/8/2677/meta
(3)Dynamical Switching of a Reaction Coordinate to Carry the System through to a Different Product State at High Energies, Hiroshi Teramoto, Mikito Toda, and Tamiki Komatsuzaki, Phys. Rev. Lett. 106, 054101 2011,
http://journals.aps.org/prl/abstract/10.1103/PhysRevLett.106.054101