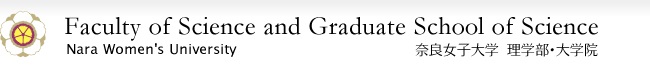Clarifying the Mechanisms of Enzyme Action with the Power of Chemistry
 [Release: November, 2016]
[Release: November, 2016]
Professor Hiroshi Fujii
Chemistry Course
Department of Chemistry, Biology, and Environmental Science
Faculty of Science
Nara Women's University
Many proteins called enzymes exist within our bodies, and they perform many chemical reactions that are necessary for biological activities. For example, enzymes are involved in digesting the foods that we eat, and also in using these ingested substances to synthesize components and energy needed for biological activities. These enzymes can induce chemical reactions under very moderate conditions and only with specific positions of specific substances. It is very difficult for chemists to chemically perform in test tubes the same reactions that enzymes perform; mimicking these processes consumes considerable energy and time, or generates undesired substances rather than the target substances. Clarifying the mechanisms catalyzed by enzymes does not just reveal the biological mechanisms at the molecular level, but also helps to understand disease mechanisms and the development of new drugs or novel substances such as artificial enzymes. Such enzyme research cannot be achieved from only a biological viewpoint, but also requires research from the viewpoint of chemistry. In my research laboratory, we use chemical knowledge to research mechanisms of enzyme action within the body. In particular, we research enzymes (metalloenzymes) that contain metal ions such as iron, copper, and nickel. Enzymes can change into even stronger enzymes by incorporating metal ions. In the absence of such metal ions in the body, health conditions may worsen, and this is closely related to the action of metal enzymes. Here, I introduce one of the latest research results of my laboratory.
Clarifying the Reaction Mechanism of the Aromatic Hydroxylation Reaction by Cytochrome P450
Within our bodies, there are enzymes that synthesize substances needed for biological activities, and conversely, those that decompose unneeded substances using molecular oxygen that we take inhale. An example is the enzyme named cytochrome P450. This enzyme is used for biological processes not just in humans, but in most organisms on the earth. As shown in Fig. 1, this enzyme has a molecule named heme in its active region (the site where the reaction occurs within the enzyme), and the iron atom in the heme is the source of the enzyme’s function. There are many types of cytochrome P450 enzymes, each with different functions.
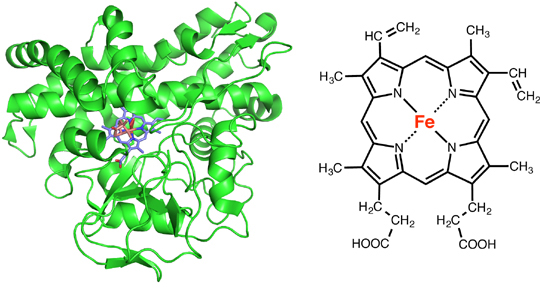
Figure 1. Complete Structure of the Cytochrome P450 Camphor (Left), and the Chemical Structure of the Active Region (Right)
(Left: The green ribbon part is protein moiety, and the light purple color shows the heme of the active site)
One of the representative reactions performed by cytochrome P450 is the hydroxylation of aromatic compounds such as benzene. An example of this hydroxylation reaction by cytochrome P450 is the process that determines the color of a flower. Many flowers get their color from a substance named anthocyanin. As shown in Fig. 2, the anthocyanin molecule is composed of three aromatic rings (A, B, C), and the color of this compound can be red, orange, violet, or blue, depending on the structure. The main factor that determines the color is the number of hydroxyl groups (OH) on the flavonoid B ring. If there is one hydroxyl group on the B ring then anthocyanin has an orange color, if two then red, and if three then blue. This hydroxylation reaction of the B ring is performed by cytochrome P450. As you can see from this example, the hydroxylation reaction of an aromatic compound by cytochrome P450 is an extremely important reaction that is indispensable for organisms. So how does the enzyme perform this reaction?
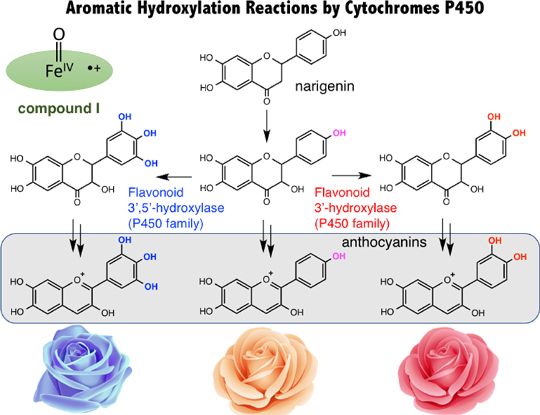
Figure 2. Mechanism that Determines the Color of a Flower
by the Aromatic Hydroxylation Reaction of Cytochrome P450
After much research, it has been clarified that the heme group in the active site of cytochrome P450 is activated by a molecular oxygen, an electron, and protons, and a very reactive active species with a high oxidation state is generated. But it remains unclear how the active species hydroxylates the aromatic compound. In my research laboratory, we have studied the mechanistic details of this hydroxylation reaction by artificially synthesized model compounds. The result, as shown in Fig. 3, is that we succeeded in experimentally presenting a new reaction mechanism. We found that in the rate-determining step of the hydroxylation reaction, an electron is transferred between the active species of the enzyme with a high oxidation state and the aromatic compound, and we revealed a that this electron transfer process is coupled with the following bond formation between the active species and the aromatic compound. This new mechanism is the only mechanism that can explain, without contradiction, many previous experimental results. A report related to this research result was published this year in the Journal of the American Chemical Society. It was also introduced with a banner on the home page for weekly publications of the Journal of the American Chemical Society. This is the result of research led by Maaya Asaka, an M2 in my research laboratory. Currently, Nami Fukui, an M1, and a fourth-year student Yuri Ishimizu, are conducting research to further develop this result.
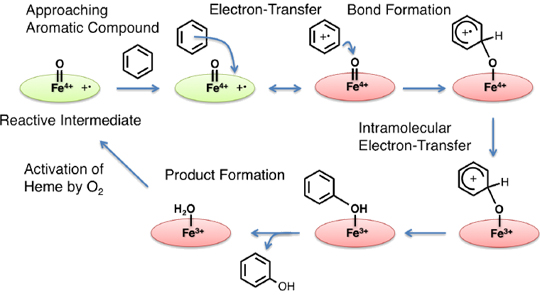
Figure 3. The Mechanism for the Aromatic Hydroxylation Reaction due to Cytochrome P450
Besides this, my laboratory has conducted research on enzymes that synthesize hypochlorous acid (the main component of bleach), which is used by white blood cells to fight bacteria that invade the body (for example, the human protein named myeloperoxidase produces bleach for antimicrobial defense), and enzymes related to the recycling of iron atoms within organisms (for example, heme oxygenase), and enzymes found in soil bacteria, which play a role in maintaining the balance of nitrogen compounds on the earth (for example, nitrite reductase). Such research is being pursued by Ikuko Araki and Sawako Yokota, both M1s, and a fourth-year student Teruyo Namba.
Since nature uses many enzymes to perform a wide variety of biological reactions, at first this may all appear extremely complicated and difficult to understand. However, I believe that among this variety there are simple and elegant rules that govern many biological reactions. In the future, by studying enzyme mechanisms, I want to continue performing research to solve the mysteries of nature.
Recent Publications Related to this Research
(1) Maaya Asaka and Hiroshi Fujii
Participation of Electron-Transfer Process in Rate-Limiting Step of Aromatic Hydroxylation Reactions by Compound I Models of Heme Enzymes
Journal of American Chemical Society 2016, 138, 8048-8051
http://pubs.acs.org/doi/pdf/10.1021/jacs.6b03223
(2) Toshitaka Matsui, Shusuke Nambu, Celia W. Goulding, Satoshi Takahashi, Hiroshi Fujii, and Masao Ikeda- Saito
Unique coupling of mono- and dioxygenase chemistries in a single active site promotes heme degradation
Proceedings of National Academy of Sciences of USA 2016, 113, 3779-3784
http://www.pnas.org/content/113/14/3779
(3) Hiroshi Fujii, Daisuke Yamaki, Takashi Ogura, and Masahiko Hada
The Functional Role of the Structure of the Dioxo-isobacteriochlorin in the Catalytic Site of Cytochrome cd1 for the Reduction of Nitrite
Chemical Science 2016, 7, 2896-2906
http://pubs.rsc.org/en/content/articlepdf/2016/sc/c5sc04825g